Overview
myTXTL Linear DNA Expression Kits contain a ready-to-use, high-performance Master Mix based on E. coli extract and a control fragment for a visual output. Protein synthesis starts within minutes after mixing the myTXTL Linear DNA Master Mix with template DNA. The kit is optimized for gene expression from linear and circular DNA (plasmid) harboring any constitutive and inducible promoter commonly used for recombinant protein production in E. coli.
- Simplicity—Easy workflow requiring minimal pipetting due to Master Mix design.
- Speed—From gene to protein within a single day.
- Power—Yields amounts sufficient for functional studies (1mg/mL per reaction).
- Versatility—Compatible with any constitutive and inducible promoter.
- Consistency—Designed for maximum robustness and reproducibility at any project scale.
- Scalable—Flexible size options, including bulk and OEM options are available
Applications
myTXTL Linear DNA Expression Kits are ideal for a range of research applications, including:
- Protein synthesis (10-200kDa and more)
- Peptide synthesis
- Bacteriophage research
- Prototyping of gene networks
- Construction of synthetic minimal cells
- Teaching biological concepts
Performance
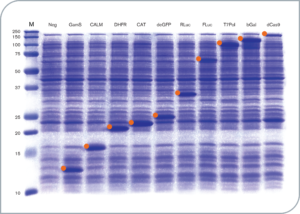
Protein production in myTXTL Cell-free Expression System (12-160 kDa)
12 µL myTXTL reactions were set up with 5-10 nM template DNA (~ 30-650 ng DNA) carrying either a P70a or T7 promoter, and incubated for 16 hours at 29°C. After acetone precipitation, 1 µL of each reaction was loaded onto an SDS-PAGE gel and stained with Coomassie. Target proteins are marked with an orange identifier. M; Protein Marker (10-250 kDa); Neg; no template DNA; GamS; Truncated nuclease inhibitor protein Gam, CALM; Calmodulin-like protein 3, DHFR; Dihydrofolate reductase, CAT; Chloramphenicol acetyl transferase, deGFP; Variant of enhanced GFP, RLuc; Renilla luciferase, FLuc; Firefly Luciferase, T7Pol; T7 RNA polymerase, bGal; Beta-Galactosidase, dCas9; dead Cas9.
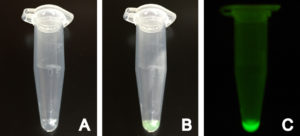
Production of positive control deGFP using myTXTL
myTXTL Sigma 70 Master Mix with deGFP control plasmid before (A) and after (B) incubation at 29°C in a 1.5 mL reaction tube. (C) Fluorescence emitted by produced deGFP under UV light.
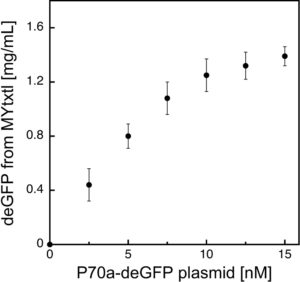
Effect of plasmid concentration on in vitro protein production
deGFP expression is regulated by the interaction of the endogenous E. coli core RNA polymerase and the primary sigma factor 70 (σ70) with the σ70-specific promoter P70a.
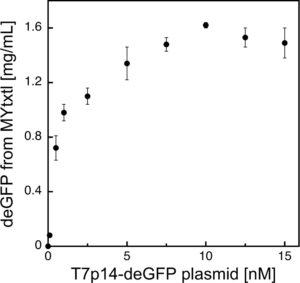
Protein synthesis in myTXTL using the T7 Expression System
deGFP expression under the control of the bacteriophage T7 promoter (PT7) is facilitated by initial expression of T7 RNA polymerase from an additional plasmid.
Resources
FAQs
- Master mix inactivation due to improper storage. The myTXTL master mix must be stored at -80°C and number of freeze-thaw cycles should be minimized.
- Improper reaction setup, Please review the recommendations to set up a myTXTL reaction in the current myTXTL handbook.
- Contamination of myTXTL reaction with nuclease. To avoid nuclease contamination, wear gloves and use nuclease-free water, sterilized tips and tubes.
Yes, scaling up the volume is possible, but above 50 uL we recommend shaking and/or switching to a reaction vessel with higher surface:volume ratio to allow proper oxygenation of the reaction mix. myTXTL reactions are very sensitive to the amount of dissolved oxygen. If using over 50 uL volume, a flat-bottomed ELISA plate or tissue culture plate may be advised along with shaking. We advise testing such a setup with our positive control deGFP plasmids or linear fragments that come with the kit. The key is to balance oxygenation and avoiding the reactions drying out due to too much surface area.
The kit used depends on the phage's genome type, if it is linear DNA, we recommend the myTXTL Linear DNA kit or myTXTL T7 Expression Kit if you need to express accessory proteins. If circular DNA or RNA, any of our kits could be used, keeping in mind that non-E. coli phages may require the addition of their host's primary transcription factor (SigA/Sigma70) or other accessory proteins to enable replication (see Emslander et al. 2022).
Batch-to-batch variation can cause varying levels of TX-TL inhibitor contamination present in the plasmid solution. Please follow our recommendations on how to prepare template plasmid DNA for TXTL reactions in the current myTXTL handbook.
A standard protocol for chemical transformation usually produces E. coli cells with a sufficient competency for plasmid intake. We recommend following the procedure described in Sambrook et al. 1989.
Consider if your recombinant protein requires co-factors like heavy metal ions or coenzymes to be functionally active. Those should be present during protein synthesis. Additionally, a low concentration of mild detergent (e.g. Triton-X-100, sodium dodecyl maltoside, or CHAPS) can be added to the reaction as well as molecular chaperones. Please note that the myTXTL system cannot introduce post-translational modifications like glycosylation or phosphorylation to your protein. Reducing the incubation temperature might help to prevent aggregation of the nascent polypeptide chain and to promote proper protein folding.
As the myTXTL platform relies on the endogenous transcription and translation machinery of E. coli, a functional gene cassette must contain a promoter that can be transcribed by E. coli RNA polymerase and associated transcription factors (primarily Sigma 70) or by a T7/T3 RNA polymerase if those polymerases are expressed from a helper plasmid (available in our Toolkit). The ribosomal binding site should also be compatible with E. coli translation machinery. For more general advice on how to construct a functional gene cassette, please refer to the current myTXTL handbook.
Yes, it is expected that protein yield resulting from linear templates is diminished compared to its circular plasmid version. A decrease of 10-30% is considered to be within the normal range.
The myTXTL Linear DNA Expression Kit is based off our myTXTL Sigma 70 Master Mix Kit, but has been further engineered to efficiently produce soluble and membrane proteins using linear DNA templates without the need for nuclease inhibitors like GamS. Simply add linear DNA template to the optimized master mix to begin protein synthesis.
deGFP is a N- and C-terminally truncated version of the reporter eGFP that is more translatable in cell-free systems. The excitation and emission spectra as well as fluorescence properties of deGFP and eGFP are identical which enables the use of commercial eGFP protein to be used in a standard curve to quantify the deGFP in the reaction.
Utilizing linear DNA templates greatly increases the speed of the design-build-test-learn cycle as laborious steps like cloning, transformation and purification are no longer necessary. This is particularly useful when working with a high number of variants of a single protein that need to be studied and validated. The reduced costs of linear DNA can also expand the sampled sequence space for protein designs compared to the plasmid format.
Due to the small reaction volume of 12 μL, it is very important to avoid condensation of water on the lid of the reaction tube, as it considerably increases the concentration of myTXTL reaction components. This can lead to poor or irreproducible kit performance. If possible, incubation in an incubator and water bath/Armor beads is best. Water facilitates a faster heat transfer than air and a water bath shows low temperature fluctuation, which should – combined with a closed environment with constant temperature surrounding the entire tube – lead to higher reproducibility and yield. A PCR thermoblock did not perform well with tubes or a multiwell plate.
Apart from standard biochemical methods like Coomassie-stained SDS-PAGE and western blot analysis, the great advantage of cell-free protein production is the open-system environment which allows the direct quantification and/or analysis of protein functionality in an activity assay without purification. Alternatively, for some activity assays downstream processing via affinity purification may be needed (if an affinity tag is present). If you choose SDS-PAGE analysis, you can either take a small sample (1-3 µL) directly from your TXTL reaction, or – to reduce background signal – precipitate proteins with TCA/acetone or ammonium-acetate/methanol following a standard protocol.
Yes! That only requires the addition of the plasmid coding for T7 RNA polymerase under transcriptional control of a σ70-specific promoter, e.g. P70a-T7rnap and possibly your inducer like IPTG if it is an inducible promoter. The optimum concentration of P70a-T7rnap is usually between 0.1 nM and 1 nM. Higher concentration normally does not increase protein yield. The more important parameter for efficient protein expression is the concentration of the plasmid that encodes for your protein of interest downstream of the T7 promoter, which will be most likely in the range of 5-20 nM.
Yes. The myTXTL master mix contains tRNAs for seven codons rarely used in E. coli to enable expression of eukaryotic proteins.
Yes! Parameters that influence protein production efficiency are:
- Gene cassette construction (promoter strength, position of affinity tag, TXTL elements)
- DNA purity
- DNA concentration
- Incubation temperature, time, and vessel
- Presence of folding helpers, chaperones, oxidizing agents
and should therefore be evaluated for optimization. Please also see our recommendations on Template Design in the current myTXTL handbook.
We share all DNA plasmid and linear fragment sequences in our portfolio to allow users to optimize their designs with respect to 5' and 3' sequences that enable optimal expression for their application, ranging from single protein expression to complex gene circuits and metabolic pathways. Plasmid or linear DNA can be ordered online from various DNA synthesis companies.
Unfortunately, this may lead to considerably decreased performance or even loss of function. To ensure highest kit performance, make sure to store the myTXTL kit or bulk master mix at -80 °C and freeze as soon as possible after usage.
For fastest sample throughput, PCR products can be directly used as template material for cell-free expression so long as the final glycerol concentration in the reaction is 0.1% or lower and the PCR reactions on average are making enough linear DNA to facilitate the protein yield needed. If a known fixed template concentration is preferred, PCR products should be subjected to a standard PCR clean up procedure with a final elution in molecular biology grade, nuclease-free water. This might also slightly boost the protein yield per reaction. Linear DNA can also be ordered and used directly in myTXTL from several commercial DNA suppliers. Please refer to our myTXTL Handbook and IDT App note for information about linear DNA template design requirements.
Unfortunately, not. However, studies have shown that supplementing cell-free systems with mixtures of reduced (GSH) and oxidized glutathione (GSSG), disulfide bond isomerase C (DsbC), protein disulfide isomerase (PDI) and/or chaperones (e.g. DnaK, DnaJ, GroEL, GroES) can promote the formation of disulfide bonds. In addition, pretreatment with iodoacetamide (IAM) to inactivate endogenous reductases which are present in the cell extract might also help (Review Article: Stech M & Kubick S, Antibodies 2015, 4, 12-33).
Yes. Due to the manufacturing process, there might be a small pellet visible. It is critical that you resuspend the myTXTL Master Mix completely before aliquoting it to set up your TXTL reaction(s).
Yes. For all plasmids containing the lambda phage promoter (P70a, P70b, P70c, P70d) it is extremely crucial to use E. coli KL740 as the transformation strain. When cultivated below 30°C, this strain over-expresses the lambda phage repressor protein Cl857 that represses P70 promoters, thus ensuring high transformation efficiency and plasmid stability. KL740 can be purchased from E. coli Genetic Stock Center (Yale) [CGSC#: 4382] or from Daicel Arbor. For all other plasmids, a standard laboratory E. coli cloning strain like JM109 or DH5alpha is sufficient.
Efficient in vitro protein production is highly dependent on the quality of the template DNA, which should be free of nucleases (DNases, RNases) and inhibitors of the TXTL machinery (e.g. EDTA, ethidium bromide, SDS, Cl- ions, ethanol). Preparation of plasmid DNA with standard commercial kits usually involves sample treatment with RNase, which may not be completely removed during downstream processing. Thus, we strongly recommend subjecting the prepared DNA to either a commercial PCR clean-up kit or standard phenol-chloroform extraction and ethanol precipitation. Ideally, template DNA is suspended in nuclease-free water. Please note, introducing Mg2+and K+ ions can compromise the kit performance, as they are extremely critical for transcription and translation, and are optimized in the master mix.
Most importantly, the excitation and emission wavelength should match the fluorescence properties of deGFP/eGFP (e.g. λEm 488 nm, λEx 535 nm). Other reader settings such as reading mode, integration time and gain value should be chosen under consideration of high well-to-well fluorescence reading reproducibility.
You are advised to keep the number of freeze-thaw-cycles to a minimum. Nevertheless, our studies have indicated that up to five freeze-thaw-cycles do not negatively influence protein production efficiency of the myTXTL Master Mix when using flash freezing in liquid nitrogen.
Sample handling and storage is mainly determined by the stability of your molecule of interest (protein, DNA, RNA) and thus optimal conditions may need to be evaluated. But to ensure sample integrity, we would recommend to either process the myTXTL reaction immediately after performing the incubation or store it at ≤ -20 °C.
Yes, myTXTL Linear DNA Expression Kit allows the use of both, linear and circular/plasmid templates.