Likely yes. Our team of scientists at Daicel Arbor Biosciences come from a range of scientific backgrounds and possess expertise in diverse applications, data analysis workflows and bioinformatics tools. It is likely that our team members have experience working with datasets and analyses that are comparable to yours, and they have a proven track record of effectively handling projects applicable to a diverse range of research fields, such as agrigenomics, non-model organism genomics, functional genomics, phylogenetics/phylogenomics, genotyping, degraded or ancient DNA, and more.
No, you do not need to have any bioinformatics expertise to work with us. We will have a consultation to understand your requirements and plan the project accordingly. Your final project report will include comprehensive methodological descriptions in sufficient detail to populate the methods section of a peer-reviewed publication.
By default, we provide a storage term of three months for project deliverables. However, we do offer the option of longer-term storage upon request, subject to an additional fee.
A standard protocol for chemical transformation usually produces E. coli cells with a sufficient competency for plasmid intake. We recommend following the procedure described in Sambrook et al. 1989.
Several factors can hinder protein synthesis in the myTXTL cell-free protein expression system, including:
- Master mix inactivation due to improper storage. The myTXTL master mix must be stored at -80°C and number of freeze-thaw cycles should be minimized.
- Improper reaction setup, Please review the recommendations to set up a myTXTL reaction in the current myTXTL manual.
- Contamination of myTXTL reaction with nuclease. To avoid nuclease contamination, wear gloves and use nuclease-free water, sterilized tips and tubes. Use deionized, nuclease-free, “molecular grade” water for myTXTL reaction setup.
Batch-to-batch variation can cause varying levels of TXTL inhibitor contamination present in the plasmid solution. Please follow our recommendations on how to prepare template plasmid DNA for TXTL reactions in the current myTXTL manual.
If you have started your project with plasmid templates for which you purchased the myTXTL Sigma 70 Master Mix, but now you want to try out linear DNA templates with the myTXTL Sigma 70 Master Mix you still have available in your lab. A simple addition of myTXTL GamS Protein to the myTXTL Sigma 70 Master Mix significantly boosts protein output from linear DNA templates in this master mix. For future work with linear DNA templates we would recommend the myTXTL Linear DNA Expression kit or myTXTL T7 Expression kit as both use the myTXTL Linear DNA Master Mix.
The kit used depends on the phage’s genome type, if it is linear DNA, we recommend the myTXTL Linear DNA kit or myTXTL T7 Expression Kit if you need to express accessory proteins. If circular DNA or RNA, any of our kits could be used, keeping in mind that non-E. coli phages may require the addition of their host’s primary transcription factor (SigA/Sigma70) or other accessory proteins to enable replication (see Emslander et al. 2022).
Our goal is to provide NGS services for your project as if you were working directly with one of your research collaborators. We strive for a transparent and cooperative approach to NGS projects, and will communicate openly with you to find the right solution for your individual research design needs. We have decades of collective experience in molecular biology, including target capture & high-throughput sequencing, and working with difficult specimens.
In order to facilitate transparent, collaborative projects, we have opted to not offer specific guarantees for target capture & sequencing service projects, since every custom project is completely unique. In science, sometimes research projects do not go 100% according to plan. However, you will only pay for the services that we actually perform. We will give every project our individual attention, and all laboratory work is performed by hand on the bench by our team of dedicated & experienced researchers. We aim to provide you with the very same dedicated laboratory attention that you & your research team would give to your own work. You can be assured that we will communicate openly with you about all aspects of your project, and will not commit to a project before first discussing all the options with you.
We offer a full suite of services for next-generation sequencing projects, on both regular specimens as well as degraded specimens such ancient DNA including:
- Extraction of DNA and RNA from fresh or degraded samples
- Short-insert DNA and RNA library preparation suitable for downstream sequencing with Illumina and similar platforms
- Long-insert DNA library preparation suitable for downstream PacBio and Nanopore platforms
- Target enrichment using myBaits catalog and custom kits
- High-throughput sequencing on Illumina and PacBio instruments
Demonstration of a range of customizable NGS library insert size options available from NGS services team at Daicel Arbor Biosciences. Our experts can help you to choose the best average insert length appropriate for your specific myBaits target capture project, whether SNP resequencing, exon capture, UCE sequencing, or more. Depending on the quality of your DNA, the nature of your desired target regions, and your planned sequencing platform and read protocol, your project may benefit from either shorter or longer NGS library sizes.
Please find the current submission guidelines and sample preparation instructions in the “Resources” section. These guidelines should be sufficient for most standard DNA or RNA extracts derived from fresh, high-quality, relatively recent specimens (e.g. DNA extracted from fresh, frozen, or alcohol-preserved tissues) or for submission of pre-made NGS libraries.
In addition, we are currently accepting most service projects for atypical or sensitive samples, including specimens with degraded or rare targets (e.g., ancient DNA, museum, archival, metagenomic, environmental, etc). However, you should first contact us with more information about your samples & research goals, so that we can determine if we are able to accept your project.
Yes we can, however we caution that there is a higher risk of sample and/or target drop-out for low-input and/or low-quality samples. In our experience across many project types, the recommended amounts outlined in the Sample Preparation Guide provide the best results.
We have outlined specific recommendations for Standard, Degraded, and Ancient DNA handling in our Sample Preparation Guide. We may recommend or request that you change the handling/package type based on the specific quantity/quality of DNA present in your samples if they do not meet the criteria for the handling type that you ordered. If you decide to proceed with a handling/package type that does not align with the quantity/quality of the DNA, you assume the risk that your sample(s) may result in little or no useful data.
We do not have a minimum sample number, but we advise that smaller projects will have a higher per-sample cost than larger projects due to the labor required to process a batch of samples and our volume discount pricing. There is no maximum sample count per project.
Please see our NGS Service Policies PDF document (available under Resources) for more detailed information.
Due to the inherently variable nature of target capture & high-throughput sequencing experiments (especially for new, untested, custom baitsets), we are unable to offer service guarantees related to turnaround time, unique read sequencing coverage depth, on-target percentage, and other experimental outcomes.
In order to facilitate transparent, collaborative projects, we have opted to not offer specific guarantees for service projects, since every custom project is completely unique. In science, sometimes research projects do not go 100% according to plan, and for target capture, it is very difficult to plan for specific sequencing outcomes without prior experimental data. However, you will only pay for the services that we actually perform, and we will not accept a project before discussing all the options with you.
You will receive updates and estimated timelines for completion of different stages of your project. If you are working within a specific deadline, we will certainly make every effort to accommodate that, though we cannot guarantee that it will be possible to finish your project within that window.
We are able to expedite a limited number of projects at any one time, please inquire if you believe your project needs to be expedited. When a project is expedited, it will skip our regular queue. We do not guarantee a specific turnaround time guarantee for expedited projects.
Yes, you can provide libraries that you have made yourself. However, we would prefer that we make the libraries so that we have direct control over the uniformity and quality of the libraries going into target capture.
If you send us libraries, they must be compatible with dual-indexed 8bp index reads, and you must make us aware of any incompatibilities within your sample set before you receive a quote.
Please confer with us to make sure that we are fully aware of the type of libraries that you are using, since it is critical to choose the correct adapter-specific blocking oligos during capture and the right amplification primers after capture.
Yes, we are currently accepting sequencing-only projects on the AVITI platform. Libraries must be compatible with the AVITI platform – see FAQ “What kind of libraries are compatible with AVITI?” for more details.
Yes. We typically have enough material to perform multiple different types of captures from a single set of libraries that we generate. Depending on your specific bait set and sample configuration, it may be possible to pool more than one different bait design in a single capture reaction. Please contact us for more information about pricing for your individual project configuration needs.
We offer extraction services for DNA and RNA for multiple sample types. Some of the options for fresh samples are tissue (including plant leaves and seeds), cells, blood, and saliva. We also have the facilities and expertise for performing DNA extraction on degraded specimens, including bones, teeth, soft tissue, FFPE, and other sample types.
If you have special or sensitive samples, we may be able to offer extraction services for your project, depending on the circumstances. Please contact us directly to inquire about special service options.
Yes. We have a small cleanroom on-site where we perform all pre-PCR procedures. We can accommodate most common ancient, museum, or historical sample types, such as bones, teeth, and soft tissue. If you have a different or unusual sample type, please contact us to discuss your project needs in greater detail.
We offer NGS services on non-hazardous DNA or RNA extracts, whether from high-quality or degraded specimens. However, your samples should still meet our standard submission requirements for your type of service project, including relevant negative controls where applicable. Otherwise, your samples may be unable to pass our quality controls, or your project could be delayed. Please review the guideline documents in the Resources section.
Please note that if you are shipping your samples internationally which require any sort of import permit, you are responsible for identifying the permit and will incur a fee for any permit applications. Additionally, we require proof of ethics clearance for working with any human or human-derived samples.
Unfortunately, not. However, studies have shown that supplementing cell-free systems with mixtures of reduced (GSH) and oxidized glutathione (GSSG), disulfide bond isomerase C (DsbC), protein disulfide isomerase (PDI) and/or chaperones (e.g. DnaK, DnaJ, GroEL, GroES) can promote the formation of disulfide bonds. In addition, pretreatment with iodoacetamide (IAM) to inactivate endogenous reductases which are present in the cell extract might also help (Review Article: Stech M & Kubick S, Antibodies 2015, 4, 12-33).
Yes, although it is not optimized or recommended for linear DNA templates. If using linear DNA with Sigma 70 MM, enhanced protein yields can be achieved by supplementing the master mix with our nuclease inhibitor GamS.
We always recommend keeping the thawing-and-freezing cycles as low as possible which might require aliquoting the original sample. However, myTXTL GamS Protein can be subjected to at least five thawing-and-freezing cycles.
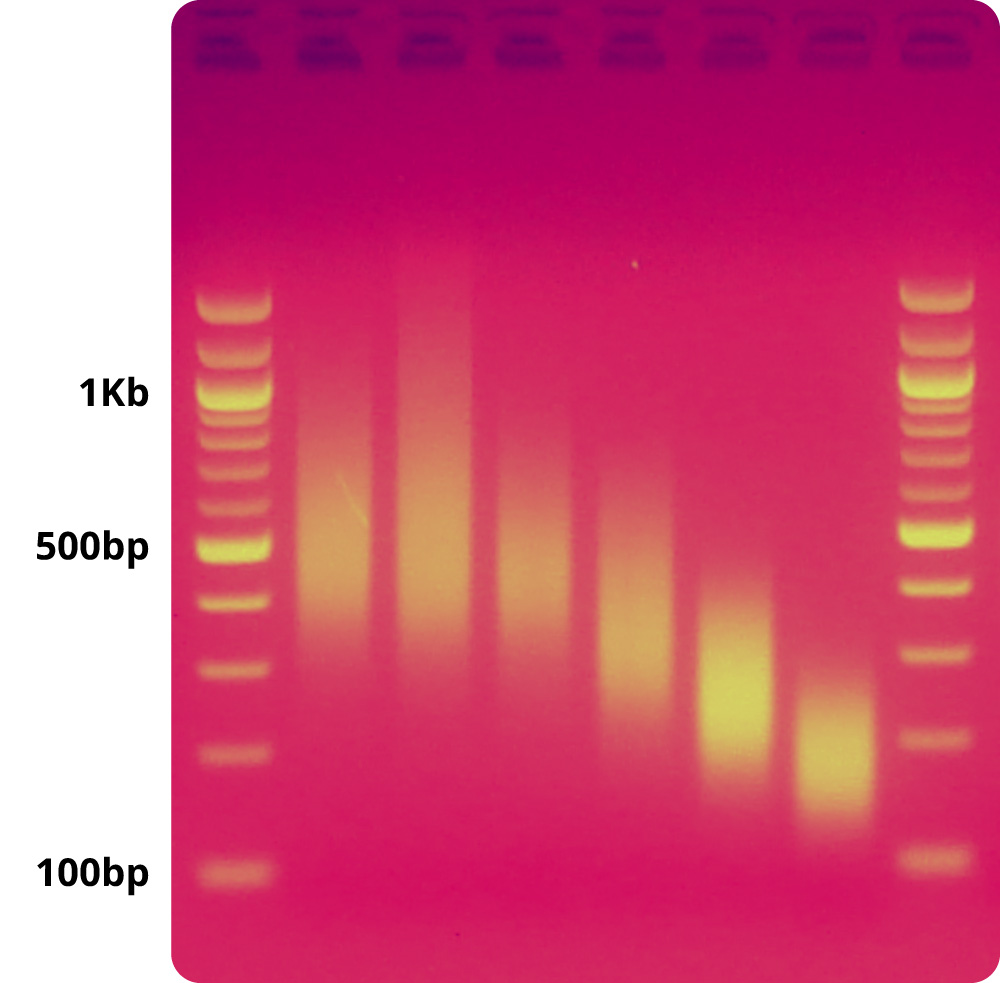
PCR products can be directly used as DNA template for cell-free expression so long as the final glycerol concentration in the reaction is 0.1% or lower and the PCR reactions on average are making enough linear DNA to facilitate the protein yield needed. If a known fixed template concentration is preferred, PCR products should be subjected to a standard PCR clean up procedure with a final elution in molecular biology grade, nuclease-free water. This might also slightly boost the protein yield per reaction. Linear DNA can also be ordered and used directly in myTXTL from several commercial DNA suppliers. Please refer to the applicable myTXTL manual and our Application Note with IDT for information about linear DNA template design requirements, or reach out to techsupport@arbor.daicel.com with questions about specific sequence recommendations.
Unfortunately, this may lead to considerably decreased performance or even loss of function. To ensure highest kit performance, make sure to store the myTXTL cell-free protein expression kit master mix at -80 °C and freeze as soon as possible after usage.

 Bluesky
Bluesky