The myTXTL Pro Kit is intended for cell-free protein expression of proteins that do not require disulfide bonds. It also has the highest yield of our two kits. The Antibody/DS Kit is intended for the expression of proteins that do contain disulfide bonds such as antibodies and enzymes. The yield of the Antibody/DS kit varies with the protein expressed, but for deGFP it is about 30-50% the yield of the Pro Kit. This is still enough protein for most downstream applications.
For work involving proteins that require disulfide bond formation we recommend our myTXTL Antibody/DS Kit. It is possible that some proteins with just 1 disulfide bond, such as VHH/Nanobodies, may express and show activity in the myTXTL Pro Kit, but generally speaking disulfide bond containing proteins will have the highest yields and best activity in the myTXTL Antibody/DS Kit.
If you were using any of our 3 original myTXTL kits, those will be discontinued in August 2024. The myTXTL Pro Kit is now the kit that will serve your needs as it has all the properties of the old kits combined into one: it supports linear and plasmid DNA as well as all E. coli promoters and also enables T7 promoter-based expression through the addition of the Pro Helper Plasmid that expresses the T7 RNA polymerase. It’s a comprehensive solution for cell-free protein expression. All Pro Kits include the Pro Helper Plasmid and a T7 deGFP Positive Control Plasmid.
If you are interested in expressing proteins that contain disulfide bonds, the myTXTL Antibody/DS Kit is what you need.
Yes, myTXTL reactions for cell-free protein expression have been conducted from 2 to 100 uL volume, but above 50 uL we recommend shaking and/or switching to a reaction vessel with higher surface:volume ratio to allow proper oxygenation of the reaction mix. myTXTL reactions are very sensitive to the amount of dissolved oxygen. If using over 50 uL volume, a flat-bottomed ELISA, deep well plate or tissue culture plate may be advised along with shaking at 650 RPM. We advise testing such a setup with one of the positive control plasmids, such as T7 deGFP. The key is to balance oxygenation and avoid the reactions drying out due to too much surface area. Please refer to the appropriate kit manual for additional guidance on scaling up reaction volume.
As the myTXTL platform relies on the endogenous transcription and translation machinery of E. coli, a functional gene cassette must contain a promoter that can be transcribed by E. coli RNA polymerase and associated transcription factors (primarily Sigma 70) or by a T7/T3 RNA polymerase if those polymerases are expressed from a helper plasmid (available in our Toolkit). The ribosomal binding site should also be compatible with E. coli translation machinery. For more general advice on how to construct a functional gene cassette, please refer to the current myTXTL handbook.
myTXTL supports all promoters used in E. coli protein expression, including promoters that rely on the endogenous E. coli transcription machinery and those that require a separate RNA polymerase such as T7. All kits come with a Helper plasmid to express the T7 RNA polymerase and enable transcription from T7 promoters. If a promoter is used that is recognized by the E. coli RNA polymerase, then this helper plasmid is not needed. If using a plasmid with an inducible promoter, such as the pET vectors, you need to add your inducer IPTG at 1 mM to the reaction and you may want to explore plasmid concentration in the range of y your inducer like IPTG if it is an inducible promoter.
All myTXTL kits support plasmid or linear DNA templates as well as mRNA.
myTXTL supports all promoters used in E. coli protein expression, including promoters that rely on the endogenous E. coli transcription machinery and those that require a separate RNA polymerase such as T7. Inducible plasmids require that the inducer be added to obtain the highest protein yield. pET (T7lac promoter) systems need 1 mM IPTG, for example, otherwise follow the guidance in the following table for inducer and plasmid concentrations in the myTXTL reaction.
We recommend the following inducer and plasmid concentrations when using some common inducible promoters:
| Promoter | Inducer | Recommended Inducer Concentration in myTXTL | Units | Recommended Plasmid Template Concentration in myTXTL | Units |
| T7lac | IPTG | 1 | mM | 10 | nM |
| TetA | aTc | 20 | µg/mL | 20 | nM |
| araBAD | L-Arabinose | 2 | % | 20 | nM |
It may be necessary at times to add components to the myTXTL system from sources that contain material of unknown effect in the myTXTL system, the following is a guide for what chemicals/reagents may be tolerated without a loss in performance:
1) Glycerol is tolerated up to 0.1% of myTXTL reaction volume
2) DMSO is tolerated up to 1% of myTXTL reaction volume
3) EDTA is tolerated up to 0.1 mM of myTXTL reaction volume
4) Tris-HCl (pH 8) is tolerated up to 50 mM of myTXTL reaction volume
5) CaCl2 is tolerated up to 1 mM of myTXTL reaction volume
6) MgCl2 is tolerated up to 1 mM of myTXTL reaction volume
7) NaCl is tolerated up to 50 mM of myTXTL reaction volume
Yes, it is expected that protein yield resulting from linear templates in cell-free protein expression is diminished compared to its circular plasmid version. A decrease of 10-30% is considered to be within the normal range for the Pro kit. This decrease tends to be minimal in the Antibody/DS Kit.
Yes, it is expected that protein yield resulting from linear templates in cell-free protein expression is diminished compared to its circular plasmid version. A decrease of 10-30% is considered to be within the normal range for the Pro kit. This decrease tends to be minimal in the Antibody/DS Kit.
myTXTL requires high quality template DNA, which should be free of nucleases (DNases, RNases) and inhibitors of the TXTL machinery (e.g. EDTA, ethidium bromide, SDS, Cl- ions, ethanol). Preparation of plasmid DNA with standard commercial kits usually involves sample treatment with RNase, which may not be completely removed during downstream processing. Thus, we strongly recommend subjecting the prepared DNA to either a commercial PCR clean-up kit or standard phenol-chloroform extraction and ethanol precipitation. Ideally, template DNA is in deionized, nuclease-free water. Please note, introducing Mg2+and K+ ions can compromise the kit performance, as they are extremely critical for transcription and translation, and are optimized in the master mix. Plasmid DNA prepared with a ZymoPure system purification kit or obtained from a commercial vendor can generally skip this extra purification step. Linear templates generated via PCR require just a single PCR Purification kit clean up (or magnetic bead cleanup) step or no clean-up if the PCR reaction is being diluted directly into the myTXTL reaction (be sure glycerol in the reaction is 0.1% or lower). See kit manual for additional recommendations.
Apart from standard biochemical methods like Coomassie-stained SDS-PAGE and western blot analysis, the great advantage of cell-free protein production is the open-system environment which allows the direct quantification and/or analysis of protein functionality in an activity assay without purification. Alternatively, for some activity assays downstream processing via affinity purification may be needed (if an affinity tag is present). If you choose SDS-PAGE analysis, you can either take a small sample (1-3 µL) directly from your TXTL reaction, or – to reduce background signal – precipitate proteins with TCA/acetone or ammonium-acetate/methanol following a standard protocol.
It is very important to avoid condensation of water on the lid of the reaction tube as it considerably increases the concentration of myTXTL reaction components and can lead to poor or irreproducible performance. Incubation in an incubator and water bath/Lab Armor beads is best. Water facilitates a faster heat transfer than air and a water bath shows low temperature fluctuation, which should – combined with a closed environment with constant temperature surrounding the entire tube – lead to higher reproducibility and yield. myTXTL reactions also work well on an Eppendorf ThermoMixer with a lid.
We rcommend using the heavy and light chain templates at an equimolar concentration of 5 nM each whether they are plasmid or linear DNA templates.
Yes! Parameters that influence protein production efficiency are:
- DNA design (promoter strength, position of affinity tag, TXTL elements)
- DNA purity
- DNA concentration
- Incubation temperature, time, and vessel
- Presence of folding helpers, chaperones, oxidizing agents
and should therefore be evaluated for optimization. Please also see our recommendations on these topics in the current myTXTL manual for your kit.
Consider if your recombinant protein requires co-factors like heavy metal ions or coenzymes to be functionally active. Those should be present during protein synthesis. Additionally, a low concentration of mild detergent (e.g. Triton-X-100, sodium dodecyl maltoside, or CHAPS) can be added to the reaction as well as molecular chaperones. Please note that the myTXTL system cannot introduce post-translational modifications like glycosylation or phosphorylation to your protein. Reducing the incubation temperature might help to prevent aggregation of the nascent polypeptide chain and to promote proper protein folding.
The positive control plasmid in the myTXTL Antibody/DS kit expresses the Gaussia Luciferase Dura protein which has 5 disulfide bonds. It is visible on an SDS-PAGE gel without purification when compared to a negative control reaction. Its activity can also be assayed using the NanoLight GLuc GLOW assay (NanoLight Technology; cat no. 320-50) and quantified using the GLuc standard sold separately (NanoLight Technology; cat no. 321-100).
The myTXTL Pro Kit is best for rebooting bacteriophage and some modifications to the reaction mix may be needed for rebooting your phage, please refer to the manual for detailed suggestions on phage rebooting.
An additional application for the myTXTL Pro kit is that it can be used to reboot bacteriophage from a genome input. Several phages have been shown to replicate in the myTXTL system and they generally benefit from the following modifications:
1) The addition of 0.3 mM each of additional dNTPs (for replication of DNA genomes) and 0.5-4% PEG 8000 can be helpful for optimal production of phage. These components along with your genome, 9 uL of Pro Master Mix, and water if needed should add up to 12 uL and be assembled in a 1.5/2 mL tube. Larger reaction volumes may require shaking as described in the manual for larger scale reactions. Incubate overnight as in our protocol.
2) You may also need to optimize the phage genome concentration if it is not already published. 0.25 nM has worked well for T7 but T4 was optimal at 1 nM. In part this is dependent on the quality of the genome. It is extremely important that the phage genome is of very high purity, free of contaminants and of high integrity.
3) Commercially available T7 phage genomic DNA can be used directly in the Pro Master Mix as a positive control (we have used DNA from BocaScientific: https://bocascientific.com/310000-86-detail).
The Library Prep Kit streamlines NGS library preparation for hybridization capture with myBaits. It includes:
- Genomic or other dsDNA is enzymatically fragmented, end-polished, and adenylated
- User-supplied adapters are ligated to the end-repaired fragments
- Ligation products are purified with SPRI beads and then amplified and SPRI’d again
- Libraries are optionally pooled and taken to myBaits capture
In addition to the upstream library preparation steps, the Library Prep Kit for myBaits includes the reagents necessary for performing the post-capture amplification step of the myBaits protocol.
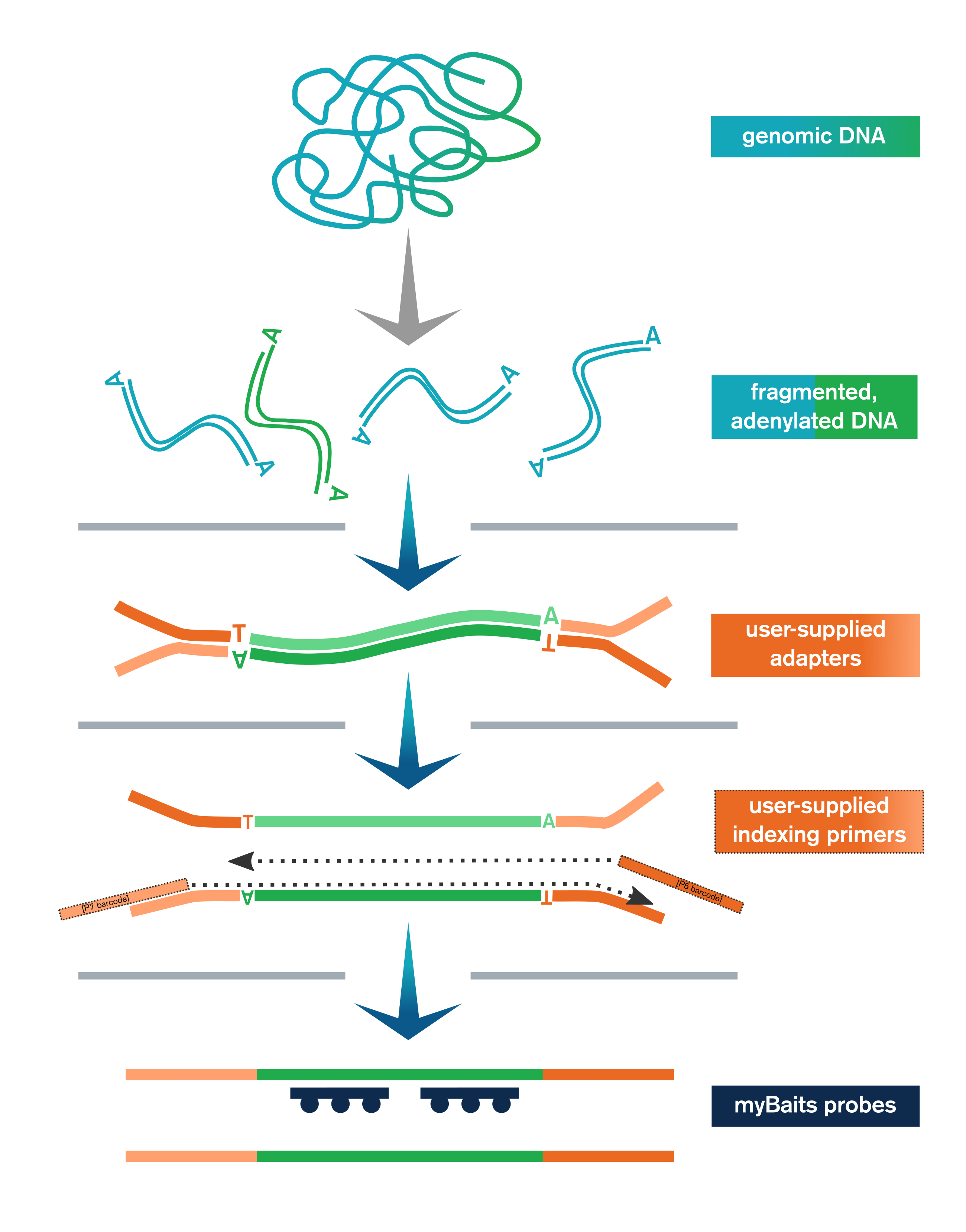
The Library Prep Kit includes reagents for key steps in NGS library preparation, including end-polishing/A-tailing, enzymatic fragmentation, ligation of user-supplied adapters, purification, and amplification (with user-supplied indexing primers, or kit-supplied universal P5/P7 primers if using full-length adapters).
In addition, the kit also provides sufficient reagents for the amplification and purification steps at the end of the myBaits capture protocol (myBaits kit sold separately).
Full details of what is included in the kit and what reagents/equipment are required to complete the protocol can be found in the kit manual.
The Library Preparation Kit for myBaits is intended to be used for the preparation of NGS libraries from DNA samples prior to hybridization capture with myBaits.
Compatible DNA samples should be or have:
- 1-500 ng genomic or other dsDNA
- <= 0.1mM EDTA storage buffer
- No viscosity or coloration
- Any length distribution; see technical recommendations for selecting frag time
Libraries made with this protocol are directly compatible with downstream myBaits enrichment (or another hybridization capture system), or alternatively can be sequenced directly in order to generate non-enriched read data.
No. myBaits hybridization capture kits have always been– and will continue to be– compatible with any user-provided upstream NGS library preparation system that is appropriate for a given project. This ensures flexibility in your targeted next-generation sequencing workflow.
However, for your convenience, we are now offering our own powerful kit for preparing NGS libraries from most types of DNA samples, which is relevant for many myBaits hybridization capture projects. This new product (Library Preparation Kit for myBaits) is intended to be used for the preparation of NGS libraries from dsDNA samples prior to hybridization capture with myBaits.
In addition to the upstream library preparation steps, the Library Prep Kit for myBaits includes the reagents necessary for performing the post-capture amplification step of the myBaits protocol.
The Library Prep Kit for myBaits is compatible with a wide range of pre-built or DIY adapters or primers. The adapters used must be T-overhang-containing double-stranded adapters. These can be either short (“stubby”) adapters OR full-length adapters that contain sample-specific barcodes. If using stubby adapters, indexing primers that add universal P5 and P7 priming sites are also required. See the kit manual for further technical information about adapters and primers.
We recommend using unique dual indexes. When selecting indexes, ensure that all libraries that you ever plan to co-enrich or co-sequence have unique index combos.
For your convenience, below are listed some commercially-available adapter and barcoding solutions designed for Illumina(R) short-read sequencing, but other vendors also supply compatible options.
Stubby adapters + indexing primers
- IDT
- xGen™ Stubby Adapter in 16 rxn (10005974) or 96 rxn (10005924)
- xGen™ UDI Primer Pairs in 8nt 16 rxn (10005975), 8nt Plate 1 (10005922), 10nt Plates 1-4 (10008052), 10nt Plates 1-8 (10008053), or 10nt Plates 1-16 (10008054)
- NEB
- NEBNext® Multiplex Oligos for Illumina® | 96 Unique Dual Index Primer Pairs – Set 1 (E6440S), Set 2 (E6442S), Set 3 (E6444S), Set 4 (E6446S), Set 5 (E6448S). (Note: Do not use the included “NEBNext Adaptor” unless you open the hairpin loop with USER treatment.)
Full-length adapters
- IDT
- xGen™ UDI-UMI Adapters in 16 rxn (10006914) or 96 rxn (10005903)
- NEB
- NEBNext® Multiplex Oligos for Illumina® | Unique Dual Index UMI Adaptors DNA – Set 1 (E7395S), Set 2 (E7874S), Set 3 (E7876S), Set 4 (E7878S)

 Bluesky
Bluesky